Human evolution didn't slow down. It accelerated
Cultural evolution has been dragging us into new environments of adaptation and selection. Far from replacing genetic evolution, it has caused the human genome to evolve faster.
Written by Peter Frost.
The dominant view is that we have evolved by changing our environment rather than ourselves. Genetic evolution has thus given way to cultural evolution. For instance, we adapt to the cold by making clothes or even a controlled environment, like a home with a fireplace. Culture has allowed us to adapt to a diverse range of circumstances, and it has diversified accordingly. Yes, we too have diversified—in shape, color, and size—but those differences are trivial. Everything else is the same, or almost.
That view has been challenged by two studies of human genetic evolution. In both cases, the research team measured the speed of evolutionary change by estimating how fast new SNPs (single nucleotide polymorphisms) have appeared on the human genome. Both teams came to the same conclusion: cultural evolution did not replace genetic evolution or even slow it down. In fact, the growing importance of culture caused the human genome to evolve faster.
John Hawks’ study
The first study was led by John Hawks, an anthropologist at the University of Wisconsin. There were two main findings:
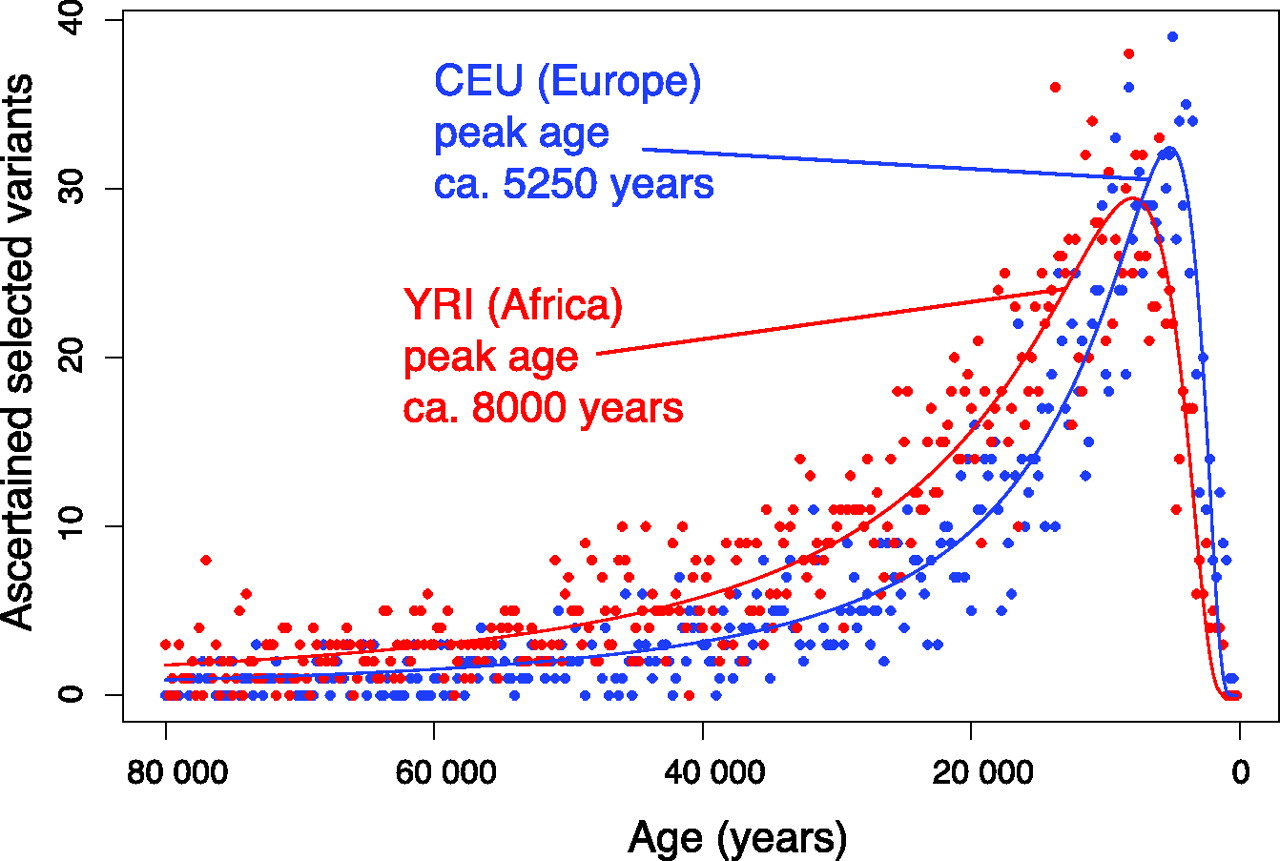
Changes to the genome accelerated more than a hundredfold when hunting and gathering gave way to farming and other cultural changes (sedentary living, growth of towns and cities, rise of social complexity, etc.).
The faster pace of genetic evolution lasted well into the time of recorded history, reaching a peak of 8,000 years ago in Africa and 5,250 years ago in Europe.
Genetic evolution began to accelerate at a time when humans had already spread from the equator to the Arctic. The impetus for acceleration came from people adapting not so much to new natural environments as to an ever-wider range of cultural environments. They were no longer just adapting to new places. They were adapting to new ways of life in the same old places.
In sum, the faster pace of cultural change made genetic change more necessary, not less so. The two modes of evolution complemented each other, with one spurring the other forward.
A bias against recent evolution?
The rate of genetic change may have actually peaked much later than the above dates of 8,000 and 5,250 years ago. As that rate increases, so does the difficulty of distinguishing between adaptive and non-adaptive genetic changes. More and more of the adaptive changes are just rising above the background noise of random mutations. If you screen out that noise, you also screen out much of recent evolution.
This sort of study is biased against recent evolution in a second way. It uses data from a limited number of present-day human groups. Thus, as you go forward from the time of early humans, you capture less and less evolutionary change within the entire human species.
Let me explain. John Hawks took his data from the HapMap project, which has samples from eleven groups:
Americans of northwest European ancestry (Utah)
Tuscans (Italy)
Han Chinese
Chinese Americans
Japanese
Mexican Americans
Gujarati (India)
African Americans
Luhya (Kenya)
Maasai (Kenya)
Yoruba (Nigeria)
Those eleven groups, and all other humans, are descended from a founder group that lived somewhere in East Africa some 100,000 years ago. As that group expanded and spread beyond Africa, it split up into new groups, which in turn split up into other new ones. Over time, more and more of the new groups would not be directly ancestral to the eleven HapMap groups; consequently, their evolutionary change cannot be inferred from the HapMap data. This problem is not specific to HapMap. It is a problem with any dataset that does not include all present-day human groups.
It seems, then, that our species has undergone more genetic change over the past ten thousand years than over the previous million. Human evolution has not been a straight line of steady change. It has been an exponential curve.
A bias against early evolution?
This sort of study may be biased not only against recent evolution but also against early evolution. A selection event is dated by measuring the decay of linkage disequilibrium (i.e., the degree to which the variants of adjacent polymorphisms are associated with each other). Early events may thus be missed if the linkage disequilibrium with adjacent variants has already completely decayed.
To find those missing selection events, you can try using a larger and denser dataset. John Hawks used a version of HapMap that was twice as large as the original one. That increase in size, however, provided only a marginal increase in the number of earlier events. He concluded that “most events (defined by the LDD test) coalescing to ages up to 80,000 years ago have been detected” (Hawks et al., 2007, p. 20754).
Ilan Libedinsky’s study
The above findings are partly supported and partly challenged by a study that came out this year. It was led by Ilan Libedinsky, a geneticist at Vrije Universiteit Amsterdam.
Like John Hawks, he used SNPs to estimate the speed of evolutionary change at different times in the past. Unlike Hawks, he used the Human Genome Dating (HGD) dataset, which is more than three times the size of HapMap. Unfortunately, he did not control his data for ethnic/geographic origin.
Ilan Libedinsky also focused on those SNPs with real phenotypic effects on mind or body. He then identified the domains of mind and body that underwent the most evolutionary change.
There were three main findings:
Human evolution went through two periods of rapid change. The first one was between 2.4 million and 280,000 years ago with a peak around 1.1 million years ago. The second period was between 280,000 and 1,700 years ago with a peak around 55,000 years ago.
The second period saw rapid evolutionary change in three domains. In order of importance, they were: (1) vision; (2) mental function; and (3) nutrient absorption, digestion, and storage. There was much less evolutionary change in metabolism, skeletal development, and the immune system.
Evolutionary change in the neocortex seems to have been more recent, on average, than evolutionary change elsewhere in the brain.

An even worse bias against recent evolution?
This study, like the one by John Hawks, shows a recent burst of accelerated evolution that extends into recorded history. Ilan Libedinsky’s study, however, shows the recent burst beginning earlier and peaking earlier. In fact, the peak occurs long before historic times, back when modern humans were spreading out of Africa.
The reason seems to be the different datasets. Although Hawks’ dataset is much smaller, it is divided equally among several human groups: two of European descent; three of East Asian descent; one of mixed European/Amerindian descent; one of South Asian descent; and four of African descent. It thus provides a fair cross-section of the human gene pool.
In contrast, Libedinsky’s dataset is mostly from people of European descent (Hawks, 2023). It thus provides a poor cross-section of the human gene pool. Moreover, Libedinsky did not look for different geographic trajectories after the Out of Africa event.
If an SNP dataset is not geographically representative, it will provide reliable data on humans only up to the time when they began spreading out of Africa some 50,000 to 60,000 years ago. From then onward, the data will come from a progressively smaller proportion of the human gene pool. Genetic evolution thus seems to peak 55,000 years ago. That “peak,” however, is illusory.
What does John Hawks think?
When I was preparing this article I asked John Hawks for his thoughts on Ilan Libedinsky’s study. His thoughts partly overlap with mine:
There is a bias in the methodology against recent adaptive changes. Such changes tend to be lower in population frequency and thus more difficult to make out against the background of random non-adaptive mutations. The result is an apparent “deficit” of recent mutations with real phenotypic consequences.
The dataset is mostly from people of European descent. It thus excludes much, if not most, of recent human evolution.
John Hawks sees evidence of recent genetic evolution being fueled, at least in part, by introgression of genes from archaic humans (Neanderthals and Denisovans). In his opinion, such introgression might explain the first period of rapid genetic change, which peaked 1.1 million years ago.
I disagree. The first peak occurred among the common ancestors of humans, Neanderthals, and Denisovans. It was only later, between 800,000 and 500,000 years ago, that the ancestors of humans parted company from those of Neanderthals and Denisovans. So the burst of rapid genetic evolution was in our direct ancestral lineage, and not in an archaic sideline.
John Hawks pursues his argument by citing Huff et al. (2010) to argue that the ancestral Homo line lost much of its genetic diversity when it underwent a roughly 50% reduction in population size between 0.9 and 1.5 million years ago. Perhaps some of that genetic diversity later returned to humans through admixture from Neanderthals or Denisovans. Perhaps, but I doubt it. Remember, the human/Neanderthal split happened long after the reduction in population size. So the presumed loss of genetic diversity would have been irretrievable, and not preserved in the Neanderthal/Denisovan line.
It’s interesting to note that the reduction in population size occurred during the first burst of rapid genetic evolution 2.4 million to 280,000 years ago. The genome was evolving unusually fast at the very time when the total human population fell from 14,500-26,000 to 8,100-8,750 individuals. I have often seen it stated that smaller populations have less potential for evolutionary change because they have less genetic variability for evolution to draw upon. But how true is that? The evolutionary biologist Ernst Mayr took the opposite view:
The factor that seems to make by far the greatest contribution to rate of speciation is population size. A species with millions of individuals has a gene pool of such enormous size that the replacement of a gene by another allele is a very slow process, and the replacement of an entire well-balanced epistatic system by another one is almost impossible. Species with large populations are, therefore, from the evolutionary point of view, highly inert. (Mayr, 1970, 348-349)
In sum, I share most of John Hawks’ major criticisms. As for his minor ones, they often amount to quibbling, and sometimes not even that. One gets the impression that he is trying to distance himself from this study and its conclusions, even though he is largely in agreement with both.
Future research?
Ilan Libedinsky used a much larger dataset and could thus identify an earlier peak of rapid evolution around 1.1 million years ago. However, with no control for geographic or ethnic origin, he could not examine evolutionary change over the last 50,000 to 60,000 years, when humans were spreading not only over the entire globe but also into new environments of living, thinking, and being.
Future researchers should divide up the HGD dataset by geographic and ethnic origin to examine the different trajectories of human evolution in Africa, Eurasia, and elsewhere. Different trajectories may have different peaks of evolutionary change during historic times. A single trajectory may even peak several times between intervening periods of stasis.
And what, exactly, was the recent evolutionary change? How much of it was change to mental function, and how much of it was change to other traits?
One thing is sure: the recent acceleration of genetic evolution was real. If it were simply some kind of glitch in the methodology, all domains of human biology would show the same acceleration, and not just a few. Mental function would have evolved at the same rate as skeletal development. Recent human evolution seems to have been driven by improvements to a relatively small number of abilities and capacities.
Yes, human evolution did accelerate, and it did so primarily to meet the challenges of culture rather than nature. Culture was largely deciding who got to live and reproduce.
Peter Frost has a PhD in anthropology from Université Laval. His main research interest is the role of sexual selection in shaping highly visible human traits, notably skin color, hair color, and eye color. Other research interests include gene-culture coevolution. Find his Newsletter here.
References
Hawks, J. (2023). Did two pulses of evolution supercharge human cognition? John Hawks Weblog, May 15. https://johnhawks.net/weblog/did-two-pulses-of-evolution-supercharge-human-cognition/
Hawks, J., Wang, E. T., Cochran, G. M., Harpending, H. C., and Moyzis, R. K. (2007). Recent acceleration of human adaptive evolution. Proceedings of the National Academy of Sciences (USA), 104, 20753-20758. https://doi.org/10.1073/pnas.0707650104
Huff, C.D., Xing, J., Rogers, A.R., and Jorde, L.B. (2010). Mobile elements reveal small population size in the ancient ancestors of Homo Sapiens. Proceedings of the National Academy of Sciences (USA), 107, 2147-2152. https://doi.org/10.1073/pnas.0909000107
Libedinsky, I., Wei, Y., de Leeuw, C., Rilling, J., Posthuma, D., and van den Heuvel, M.P. (2023). Genetic timeline of human brain and cognitive traits. February 6. bioRxiv https://doi.org/10.1101/2023.02.05.525539
Mayr, E. (1970). Populations, Species, and Evolution. Cambridge (Mass.): Belknap Press.




According to my understanding, evolution requires selection, not just genetic mutation. "Changes to the genome accelerated more than a hundredfold when...". Changes to the genome just means there is more genetic mutation because there are more people. Evolution needs more than that, it needs strong levels of selection for certain mutations.
This is admitted in the article: "A species with millions of individuals has a gene pool of such enormous size that the replacement of a gene by another allele is a very slow process". Right now, we have an enormous gene pool. Right now, there is only weak selection taking place. I am skeptical that evolution has accelerated at all since hunter-gatherer times.
Windsor Swan,
Yes, there are more mutations with a larger population, but only selection can drive those mutations to fixation. You actually make that point, so I'm puzzled by your conclusion: "I am skeptical that evolution has accelerated at all since hunter-gatherer times." If the human genome has been undergoing a faster rate of change, that would surely indicate stronger selection, and not just more mutations.
Right now, there is plenty of selection, but it's for mental traits like extraversion and neuroticism and for physical traits like obesity.
See my article: https://peterfrost.substack.com/p/the-great-decline
Shane Simonsen,
Genetic variability (or rather the lack thereof) has not been a major constraint on human evolution, even in relatively small populations. There has been considerable cognitive evolution among the Parsis and the Ashkenazim, yet both groups were quite small in number during most of their existence.
Matt Osborne,
No, the acceleration began much earlier, and we're still not sure when it ended. In fact, we're just starting to understand human evolution during historic times, thanks to ancient DNA data. In general, it looks like imperialism is bad for cognitive evolution (collapse of fertility among the upper and middle classes, increase in polygyny and female hypergamy, slavery and globalization of labor markets, etc.).
Y chromosome bottlenecks are typical of highly polygynous societies, and such societies tend to be evolutionarily static.