Written by Peter Frost.
Toxoplasma gondii is a tiny protozoan. It’s also a parasite. Like a surprising number of parasites, it can infiltrate brain tissue and make its host behave in ways that help it spread to new hosts.
For example, an infected rat is attracted to the smell of cat urine and ends up getting eaten by a cat, the only host in which T. gondii can sexually reproduce. Once inside its new host, the parasite targets the amygdala—an area of the brain that stores emotional memories. Autopsies of infected rodents show twice the density of T. gondii cysts in the amygdala as in other brain regions (Vyas et al., 2007). Primates can also get infected, and the behavioral changes are similar. Infected chimpanzees love the smell of leopard urine (Poirotte, 2016).
Yes, humans too can get infected—between 13 and 43% of adults 25 to 50 years of age in Europe, with lower rates in North America and higher ones in Brazil and Africa (Friesema et al., 2025). Some strains seem to have adapted specifically to human hosts. Of the three main lineages, Type II strains are the most common ones in our species (Hosseini et al., 2019; Xiao & Yolken, 2015).
Like other animal hosts, infected humans undergo mental and behavioral changes, with men becoming more jealous and women more easy-going. Infected individuals of both sexes respond more slowly to threats, as shown by a higher risk of traffic accidents and longer reaction time. The last finding reveals the direction of causality: the longer you have been infected, the slower you react. It’s not that slow reaction time increases the risk of infection (Flegr et al., 2005; Havlíček et al., 2001; Latifi et al., 2025).
Do these effects help T. gondii somehow? The question is hard to answer because we cannot experiment with humans as we do with lab animals. Humans also live a long time. Decades may pass between the initial infection and the ultimate pay-off for the parasite (Cochran et al., 2000; Frost, 2020).
Nonetheless, a group of Czech researchers is convinced that some strains of T. gondii have evolved the ability to manipulate human behavior, specifically sexual behavior. Male hosts are inclined not only to have more sexual partners but also to engage in forms of sex that transmit the parasite more efficiently, i.e., into the oral cavity or anal canal of a new host. This sexual lifestyle doesn’t benefit the host reproductively. In fact, fertility is reduced through lower sperm counts and lower sperm motility (Hlaváčová et al., 2021; Kaňková et al., 2020).
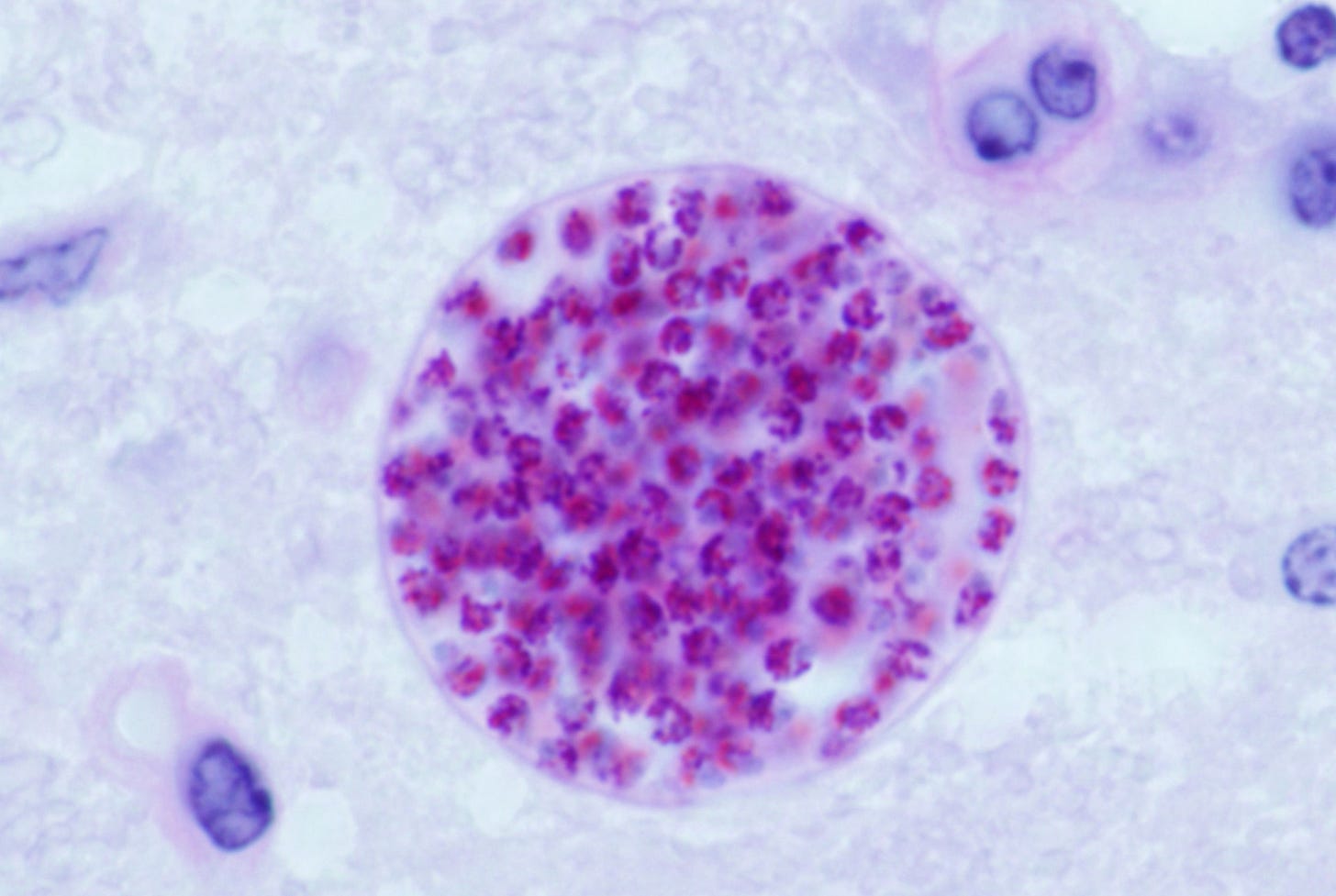
We know that T. gondii is present in male ejaculate as cysts containing thousands of spores. We also know that the cysts are spread from one body to another through fellatio, anal sex or vaginal sex. They cannot be spread from female to male.
Sexual transmission, particularly from a male host, is indicated by several lines of evidence:
In heterosexual couples, an infected male partner increases the female partner’s risk of infection, but an infected female partner does not increase the male partner’s risk of infection.
In women, the risk of infection correlates with sex work, unprotected sexual intercourse before pregnancy and history of genital injuries. In men it correlates with sexual promiscuity, and in male homosexuals with unprotected anal sex.
Seroprevalence of T. gondii is higher in fellating individuals of either sex than in non-fellating controls.
Seroprevalence is higher in homosexuals and promiscuous individuals.
Seroprevalence is positively correlated with the prevalence of STDs across countries, including HIV.
Seroprevalence is higher in women than in men. The gender difference emerges in the 10–14 age group and peaks among 20–39 year olds (Flegr et al., 2005; Latifi et al., 2025).
T. gondii seems to manipulate humans not only behaviorally but also physically. Infected men are taller than uninfected men, and women rate them as being more dominant and masculine. Infected men also have lower second-to-fourth digit ratios, a sign of greater exposure to male hormones (Flegr et al., 2005; Hodková et al., 2007; Latifi et al., 2025). This physical manipulation is in line with a strategy of making infected males more attractive to prospective hosts.
Host manipulation seems to be the last stage of a coevolution that began when T. gondii first entered a human population:
Entry into a human population, apparently via contact with cats. Note that humans began to coexist with cats some 10,000 years ago in the Middle East (Galal et al., 2022).
Passive sexual transmission. Natural selection favored strains that could adapt to a life cycle within human bodies. At this stage, the parasite was spread passively from one host to another via physical contact, often sexual intercourse.
Active sexual transmission. Natural selection now favored those strains that could modify human behavior to facilitate sexual transmission.
We are only beginning to realize that T. gondii is sexually transmitted. At present, medical research is biased toward obvious STDs, i.e., those that cause observable symptoms that occur soon after infection and progress quickly. But these are not the characteristics of a parasite that infects long-lived species, like our own. In such species, harming the host would reduce the parasite’s prospects for infecting more individuals. Its prospects are better if it remains discreet and does as little harm as possible, at least as long as its host is still useful.
Indeed, many diseases of old age may be due not to old age itself but to long-present parasites that no longer have anything to lose. From the parasite’s standpoint, the host has served its purpose and no penalty is incurred by harming it.
Are other parasites messing with our sex lives?
T. gondii may not be the only parasite that manipulates human behavior. We are simply too good to pass up. Our complex neural circuitry, our social nature and our dense populations make us ideal vectors. Even if a parasite initially has no ability to manipulate human behavior, such an ability can evolve soon enough. All of the right conditions are in place.
The following are several disorders that may be caused by behavior-manipulating parasites. Again, the disorder could be the final destructive stage of a long-present parasite that no longer has to keep a low profile.
HIV-Associated Neurocognitive Disorders (HAND). Although HIV-associated neurocognitive disorders are widely attributed to HIV, the relationship between the two remains circumstantial. In fact, HAND occurs even in individuals who have lost all detectable traces of HIV through antiretroviral therapy. One study found that 21% of them nonetheless went on to develop dementia (McArthur & Brew, 2010).
The causal agent seems to function as an AIDS cofactor—not by making an HIV infection worse but by increasing the host’s appetite for sexual behaviors that increase the risk of HIV infection.
This cofactor may be among the opportunistic infections that are blamed on the host’s compromised immune system. While such infections primarily target the lungs, the brain is the second most-common target (Masliah et al., 2000; Jellinger et al., 2000).
The existence of a brain-manipulating cofactor is consistent with the profile of AIDS victims in a study from Bologna, Italy. In that country, AIDS is transmitted mainly via intravenous drug use, yet transmission via homosexuality/bisexuality is ten times more often associated with cognitive impairment (De Ronchi et al., 2002; Wikipedia, 2025). Certainly, AIDS does impair cognition, as shown by an association between low white cell counts and HAND in the Bologna study. But some cofactor must also be impairing cognition via the homosexuality/bisexuality route.
The cofactor might be the hepatitis C virus. In a large cohort of people with HIV, the risk of HAND was higher among those with antibodies to hepatitis C (Mastrorosa et al., 2023).
Candida albicans (vaginal yeast). Candida has been implicated in several neurodegenerative diseases, notably Alzheimer's, multiple sclerosis and autism spectrum disorders. It has thus evolved the ability to cross the blood-brain barrier and adhere to tissues in the central nervous system, particularly white matter. Because of antibody cross-reactivity, the actual Candida species is difficult to identify, though C. albicans is the main suspect (Denaro et al., 1995; Jong et al., 2001).
Keep in mind that C. albicans encompasses many strains that differ substantially from each other in various ways: single nucleotide polymorphisms, inversions, copy number changes, loss of heterozygosity and whole or partial chromosomal aneuploidies. At least one of these changes is responsible for altering the balance between commensalism and pathogenesis (Hirakawa et al., 2015; see also Tian et al., 2021).
C. albicans can colonize many body sites, but some strains have adapted specifically to the vagina. A few are responsible for vulvovaginal candidiasis (commonly known as yeast infection), which affects 70 to 75% of sexually active women at least once and 5 to 8% recurrently (Li et al., 2008). In China, two strains account for almost 60% of yeast infection cases, with neither being present at extragenital sites (Li et al. 2008).
Sexual transmission is indicated by several lines of evidence:
Once a vaginal infection develops, it can spread to the male partner’s glans penis via vaginal sex or to his oral cavity via cunnilingus (Li et al., 2008; Schmid et al., 1995).
The same strains seem to infect both the vagina and the glans penis (Ge et al., 2012; see also Tian et al., 2021).
In both men and women, genital yeast infections are associated with a higher number of sexual partners (Warszawski et al., 1996).
There seems to have been selection for sexual transmissibility, particularly via cunnilingus. Vaginal strains adhere better than other strains to saliva-coated surfaces (Schmid et al. 1995).
Transmission is woman to man and not man to woman. Infected people do not have higher rates of penis-in-vagina sex, but they do have higher rates of oral sex, notably cunnilingus (Hellberg et al., 1995; Reed et al., 2003). C. albicans in the male partner’s oral cavity does not predict recurrence of yeast infection in the female partner (Reed et al., 2003), and treatment of the male partner with antifungals does not prevent recurrence of yeast infection in the female partner (Bisschop et al., 1986; Buch et al. 1982). It looks as if this parasite acts on the female partner, specifically on her sexual behavior—by weakening her inhibitions and by increasing vaginal contact with her partner's body, particularly his mouth.
Infection seems to occur in three stages:
The parasite colonizes a woman's vagina as a commensal with low virulence and no yeast infection. The ensuing period of latency may last a long time.
Other sites are then colonized in the host's body, including brain sites that influence sexual behavior.
Once secondary colonization is complete, the area of primary colonization enters a highly infectious stage, i.e., yeast infection. The pathogen can now spread to a male partner.
Multiple sclerosis. This disease causes lesions throughout the nervous system, but a recurring symptom is impairment of social cognition through damage to the limbic system, particularly the amygdala (Batista et al., 2016; Hillyer et al., 2023; Meyer-Arndt et al., 2022). As we have seen with T. gondii, this brain region is a primary target for manipulation of behavior.
Multiple sclerosis seems to be associated with a fungal parasite that infiltrates the brain and nervous system, perhaps a form of Candida. In people with the disease, this association is indicated by:
Antibodies against various Candida species.
High levels of immune defense proteins that bind to mannoproteins, which are ubiquitous in fungal cell walls but rare in bacterial and mammalian cell walls.
High levels of chitotriosidase, which the immune system produces to destroy chitin—a component of fungal cell walls but not of bacterial and mammalian cell walls.
Successful treatment of multiple sclerosis with a fungicide, dimethyl fumarate (Benito-Leon & Laurence, 2017).
MS seems to be sexually transmitted. It is rare before puberty, two to three times more common in women, more common in women taking oral contraceptives. and associated with smoking—a sociological marker of sexual activity among women. It is also associated with herpes simplex virus type 2 (Benito-Leon & Laurence, 2017; Golden & Voskuhl, 2017; Hawkes, 2002).
Unlike the unknown parasite that may cause HAND, this one seems to begin doing harm early in adulthood, at around 30 years of age, perhaps because this is when female hosts tend to abandon their multi-partner lifestyle. Consequently, the parasite no longer has anything to gain from keeping its host healthy. The mean age of onset for MS has nonetheless risen in recent decades (Golden & Voskuhl, 2017; Romero-Pinel et al., 2022).
Homosexuality and paraphilias
Let’s go beyond medical disorders and consider alternative sexual lifestyles. Could they also be caused, to some degree, by unknown parasites?
More than two decades ago, Greg Cochran argued for the existence of a “gay germ,” i.e., a pathogen that modifies sexual orientation to increase the number of hosts it can infect via sexual relations (Cochran et al., 2000). He argued that exclusive male homosexuality cannot be primarily genetic because of the high fitness cost and because twin studies indicate a heritability of only 20%. Instead, it may be caused by a pathogen that targets the limbic system. “Indeed, anecdotal reports indicate that changes in human sexual orientation have occurred following changes in the limbic area due to trauma or infection.” The pathogen may be exploiting a niche that provides more opportunities for sexual transmission. “Homosexual behavior could facilitate spread because of the larger numbers of partners homosexual males may have on average, relative to heterosexual males” (Cochran et al., 2000).
Other pathogens may be responsible for certain paraphilias, such as the cuckold fetish—whereby a male host abandons mate guarding and even feels pleasure at the prospect of being cuckolded. This fetish is absent from Greco-Roman literature, which nonetheless attests to a wide range of alternative sexualities. The earliest references come from 17th century England, particularly among merchants. The date and milieu point to an external source—most likely, slaves imported by English traders from West Africa. Because of the region’s high polygyny rate, conditions were ideal for the evolution of STDs that could spread by inhibiting, or even inversing, male sexual jealousy. Keep in mind that the barriers to transmission were already low. The head of household was generally an older man who could not satisfy all his wives. And his wives were often solicited by young single men, as inevitably happens under polygyny (Frost, 2023).
This point has been made by anthropologist Pierre van den Berghe:
The temporary celibacy of young men in polygynous societies is rarely absolute, however. While it often postpones the establishment of a stable pair-bond and the procreation of children, it often does not preclude dalliance with unmarried girls, adultery with younger wives of older men, or the rape or seduction of women conquered in warfare. Thus, what sometimes looks like temporary celibacy is, in fact, temporary promiscuity. (van den Berghe, 1979, pp. 50-51)
Polygynous households were thus vulnerable to any STD that could overcome the already low barriers to entry. Once this niche was colonized, selection favored those strains that could lower the barriers further.
Conclusion
In theory, all sexual pathogens should be under selection to manipulate host behavior. Their continued existence depends on how the host behaves, and even a slight behavioral change could significantly improve their ability to infect new hosts.
Behavioral manipulation may thus help such pathogens do a better job of exploiting their niche. Some simply make their host less discriminating, as appears to be the case with Toxoplasma gondii. Others reorient their host’s sexual orientation. Still others cause sexual fetishes that are otherwise difficult to explain with evolutionary theory.
The possible existence of such parasites, and their strategies for host recruitment, may open up new avenues of research—not only on STDs but on human behavior too.
Peter Frost has a PhD in anthropology from Université Laval. His main research interest is the role of sexual selection in shaping highly visible human traits. Find his newsletter here.
Support Aporia with a paid subscription:
You can also follow us on Twitter.
References
Batista, S., d’Almeida, O. C., Afonso, A., Freitas, S., Macário, C., Sousa, L., ... & Cunha, L. (2017). Impairment of social cognition in multiple sclerosis: Amygdala atrophy is the main predictor. Multiple Sclerosis Journal, 23(10), 1358-1366. https://doi.org/10.1177/1352458516680750
Benito-Leon, J., & Laurence, M. (2017). The Role of Fungi in the Etiology of Multiple Sclerosis, Frontiers in Neurology 16 October https://doi.org/10.3389/fneur.2017.00535
Bisschop, M. P. J. M., Merkus, J. M. W. M., Scheygrond, H., & Van Cutsem, J. (1986). Co‐treatment of the male partner in vaginal candidosis: a double‐blind randomized control study. BJOG: An International Journal of Obstetrics & Gynaecology, 93(1), 79-81. https://doi.org/10.1111/j.1471-0528.1986.tb07818.x
Buch, A., & Christensen, E. S. (1982). Treatment of vaginal candidosis with natamycin and effect of treating the partner at the same time. Acta Obstetricia et Gynecologica Scandinavica, 61(5), 393-396. https://doi.org/10.3109/00016348209156578
Cochran, G.M., Ewald, P.W., & Cochran, K.D. (2000). Infectious Causation of Disease: An Evolutionary Perspective. Perspectives in Biology and Medicine, 43(3), 406-448. https://doi.org/10.1353/pbm.2000.0016
De Ronchi, D., Faranca, I., Berardi, D., Scudellari, P., Borderi, M., Manfredi, R., & Fratiglioni, L. (2002). Risk Factors for Cognitive Impairment in HIV-1-Infected Persons with Different Risk Behaviors. Archives of Neurology, 59(5), 812-818. https://doi.org/10.1001/archneur.59.5.812
Denaro, F.J., López-Ribot, J.L., & Chaffin, W.L. (1995). Adhesion of Candida albicans to Brain Tissue of Macaca mulata in an Ex Vivo Assay. Infection and Immunity , 63(9), 3438-3441. https://doi.org/10.1128/iai.63.9.3438-3441.1995
Flegr J. (2013). Influence of latent Toxoplasma infection on human personality, physiology and morphology: pros and cons of the Toxoplasma-human model in studying the manipulation hypothesis. Journal of Experimental Biology, 216, 127–133. https://doi.org/10.1242/jeb.073635
Flegr, J., Hrŭsková, M., Hodný, Z., Novotná, M., & Hanušová, J. (2005). Body height, body mass index, waist-hip ratio, fluctuating asymmetry and second to fourth digit ratio in subjects with latent toxoplasmosis. Parasitology, 130(6), 621-628. https://doi.org/10.1017/S0031182005007316
Friesema, I. H., Waap, H., Swart, A., Györke, A., Le Roux, D., Evangelista, F. M., ... & Opsteegh, M. (2025). Systematic review and modelling of Toxoplasma gondii seroprevalence in humans, Europe, 2000 to 2021. Eurosurveillance, 30(34), 2500069. https://doi.org/10.2807/1560-7917.es.2025.30.34.2500069
Frost, P. (2023). Cuckoldry: Sexual Fantasies. In: Shackelford, T.K. (ed.) Encyclopedia of Sexual Psychology and Behavior. Springer. https://doi.org/10.1007/978-3-031-08956-5_757-1
Frost, P. (2020). Are Fungal Pathogens Manipulating Human Behavior? Perspectives in Biology and Medicine, 63(4), 591-601. https://doi.org/10.1353/pbm.2020.0059
Galal, L., Ariey, F., Gouilh, M. A., Dardé, M. L., Hamidović, A., Letourneur, F., ... & Mercier, A. (2022). A unique Toxoplasma gondii haplotype accompanied the global expansion of cats. Nature Communications, 13(1), 5778. https://doi.org/10.1038/s41467-022-33556-7
Ge, S. H., Xie, J., Xu, J., Li, J., Li, D. M., Zong, L. L., ... & Bai, F. Y. (2012). Prevalence of specific and phylogenetically closely related genotypes in the population of Candida albicans associated with genital candidiasis in China. Fungal Genetics and Biology, 49(1), 86-93. https://doi.org/10.1016/j.fgb.2011.10.006
Golden, L.C., & Voskuhl, R. (2017). The Importance of Studying Sex Differences in Disease: The example of Multiple Sclerosis. Journal of Neuroscience Research, 95(1-2), 633-643. https://doi.org/10.1002/jnr.23955
Havlíček, J., Gasová, Z. G., Smith, A. P., Zvára, K. & Flegr, J. (2001). Decrease of psychomotor performance in subjects with latent ‘asymptomatic’ toxoplasmosis. Parasitology, 122, 515-520. https://doi.org/10.1017/S0031182001007624
Hawkes, C. H. (2002). Is multiple sclerosis a sexually transmitted infection?. Journal of Neurology, Neurosurgery & Psychiatry, 73(4), 439-443. https://doi.org/10.1136/jnnp.73.4.439
Hellberg, D., Zdolsek, B., Nilsson, S., & Mårdh, P. A. (1995). Sexual behavior of women with repeated episodes of vulvovaginal candidiasis. European Journal of Epidemiology, 11, 575-579. https://doi.org/10.1007/BF01719311
Hillyer, A., Sharma, M., Kuurstra, A., Rosehart, H., Menon, R., & Morrow, S. A. (2023). Association between limbic system lesions and anxiety in persons with multiple sclerosis. Multiple Sclerosis and Related Disorders, 79, 105021. https://doi.org/10.1016/j.msard.2023.105021
Hirakawa, M. P., Martinez, D. A., Sakthikumar, S., Anderson, M. Z., Berlin, A., Gujja, S., ... & Cuomo, C. A. (2015). Genetic and phenotypic intra-species variation in Candida albicans. Genome research, 25(3), 413-425. http://www.genome.org/cgi/doi/10.1101/gr.174623.114
Hlaváčová J., Flegr J., Řežábek K., Calda P., Kaňková Š. (2021). Association between latent toxoplasmosis and fertility parameters of men. Andrology, 9, 854–862. https://doi.org/10.1111/andr.12969
Hodková H., Kolbeková P., Skallová A., Lindová J., & Flegr J. (2007). Higher perceived dominance in Toxoplasma infected men – a new evidence for role of increased level of testosterone in toxoplasmosis-associated changes in human behaviour. Neuroendocrinology Letters, 28, 110–114.
Hosseini, S.A., Amouei, A., Sharif, M., et al. (2019). Human toxoplasmosis: a systematic review for genetic diversity of Toxoplasma gondii in clinical samples. Epidemiology and Infection, 147:e36. https://doi.org/10.1017/S0950268818002947
Jellinger, K.A., Setinek, U., Drlicek, M., Böhm, G., Steurer, A., & Lintner, F. (2000). Neuropathology and General Autopsy Findings in AIDS during the Last 15 Years. Acta Neuropathologica, 100(2), 213-220. https://doi.org/10.1007/s004010000245
Jong, A.Y., Stins, M.F., Huang, S-H., Chen, S.H.M., & Kim, K.S. (2001). Traversal of Candida albicans across Human Blood-Brain Barrier In Vitro. Infection and Immunity, 69(7), 4536-4544. https://doi.org/10.1128/iai.69.7.4536-4544.2001
Kaňková Š., Hlaváčová J., Flegr J. (2020). Oral sex: a new, and possibly the most dangerous, route of toxoplasmosis transmission. Medical Hypotheses, 141, 109725. https://doi.org/10.1016/j.mehy.2020.109725
Kurtzke, J. F., Hyllested, K., Heltberg, A., & Olsen, A. (1993). Multiple sclerosis in the Faroe Islands. 5. The occurrence of the fourth epidemic as validation of transmission. Acta neurologica scandinavica, 88(3), 161-173.
Latifi, A., Flegr, J., & Kaňková, Š. (2025). Re-assessing host manipulation in Toxoplasma: the underexplored role of sexual transmission–evidence, mechanisms, implications. Folia Parasitologica, 72, 015. http://dx.doi.org/10.14411/fp.2025.015
Li, J., Fan, S. R., Liu, X. P., Li, D. M., Nie, Z. H., Li, F., ... & Bai, F. Y. (2008). Biased genotype distributions of Candida albicans strains associated with vulvovaginal candidosis and candidal balanoposthitis in China. Clinical Infectious Diseases, 47(9), 1119-1125. https://doi.org/10.1086/592249
Masliah, E., DeTeresa, R.M., Mallory, M.E., & Hansen, L.A. (2000). Changes in Pathological Findings at Autopsy in AIDS Cases for the Last 15 Years. AIDS, 14 (1), 69-74. https://doi.org/10.1097/00002030-200001070-00008
Mastrorosa, I., Pinnetti, C., Brita, A. C., Mondi, A., Lorenzini, P., Del Duca, G., ... & Antinori, A. (2023). Declining prevalence of human immunodeficiency virus (HIV)–associated neurocognitive disorders in recent years and associated factors in a large cohort of antiretroviral therapy–treated individuals with HIV. Clinical Infectious Diseases, 76(3), e629-e637. https://doi.org/10.1093/cid/ciac658
McArthur, J.C., & Brew, B.J. (2010). HIV-associated Neurocognitive Disorders: Is There a Hidden Epidemic? AIDS, 24(9), 1367-1370. https://doi.org/10.1097/qad.0b013e3283391d56
Meyer-Arndt, L., Kuchling, J., Brasanac, J., Hermann, A., Asseyer, S., Bellmann-Strobl, J., ... & Weygandt, M. (2022). Prefrontal-amygdala emotion regulation and depression in multiple sclerosis. Brain Communications, 4(3), fcac152. https://doi.org/10.1093/braincomms/fcac152
Poirotte C., Kappeler P.M., Ngoubangoye B., Bourgeois S., Moussodji M., & Charpentier M.J.E. (2016). Morbid attraction to leopard urine in Toxoplasma-infected chimpanzees. Current Biology, 26, R98–R99. https://doi.org/10.1016/j.cub.2015.12.020
Reed, B. D., Zazove, P., Pierson, C. L., Gorenflo, D. W., & Horrocks, J. (2003). Candida transmission and sexual behaviors as risks for a repeat episode of Candida vulvovaginitis. Journal of Women's Health, 12(10), 979-989. https://doi.org/10.1089/154099903322643901
Romero-Pinel, L., Bau, L., Matas, E., León, I., Muñoz-Vendrell, A., Arroyo, P., ... & Martínez-Yélamos, S. (2022). The age at onset of relapsing-remitting multiple sclerosis has increased over the last five decades. Multiple Sclerosis and Related Disorders, 68, 104103. https://doi.org/10.1016/j.msard.2022.104103
Schmid, J., Hunter, P. R., White, G. C., Nand, A. K., & Cannon, R. D. (1995). Physiological traits associated with success of Candida albicans strains as commensal colonizers and pathogens. Journal of Clinical Microbiology, 33(11), 2920-2926. https://doi.org/10.1128/jcm.33.11.2920-2926.1995
Schmid, J., Rotman, M., Reed, B., Pierson, C. L., & Soll, D. R. (1993). Genetic similarity of Candida albicans strains from vaginitis patients and their partners. Journal of clinical microbiology, 31(1), 39-46. https://doi.org/10.1128/jcm.31.1.39-46.1993
Tian, J. Y., Yang, Y. G., Chen, S., Teng, Y., & Li, X. Z. (2021). Genetic diversity and molecular epidemiology of Candida albicans from vulvovaginal candidiasis patients. Infection, Genetics and Evolution, 92, 104893. https://doi.org/10.1016/j.meegid.2021.104893van den Berghe, P. L. (1979). Human Family Systems. An Evolutionary View. New York: Elsevier.
Vyas, A., Kim, S. K., Giacomini, N., Boothroyd, J. C., & Sapolsky, R. M. (2007). Behavioral changes induced by Toxoplasma infection of rodents are highly specific to aversion of cat odors. Proceedings of the National Academy of Sciences, 104(15), 6442-6447. https://doi.org/10.1073/pnas.0608310104
Warszawski, J., Meyer, L., & Bajos, N. (1996). Is genital mycosis associated with HIV risk behaviors among heterosexuals? ACSF Investigators. Analyse des comportements sexuels en France. American Journal of Public Health, 86(8_Pt_1), 1108-1111. https://doi.org/10.2105/AJPH.86.8_Pt_1.1108
Wikipedia. (2025). “HIV/AIDS Public Health Campaigns in Italy” https://en.wikipedia.org/wiki/HIV/AIDS_Public_Health_Campaigns_in_Italy
Xiao, J., & Yolken, R. H. (2015). Strain hypothesis of Toxoplasma gondii infection on the outcome of human diseases. Acta Physiologica, 213(4), 828. https://doi.org/10.1111/apha.12458




"Your sacred identity is literally the symptom of an infectious brain parasite" would be a hell of a pill for the left to swallow, but it certainly looks like a fascinating avenue of research.
Those little guys are creepy as all hell.
Something similar is the fungus that turns ants into suicidal robots.
https://en.wikipedia.org/wiki/Ophiocordyceps_unilateralis